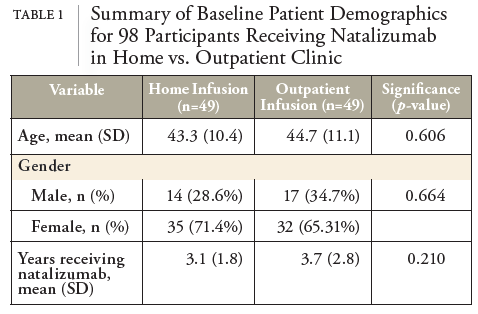
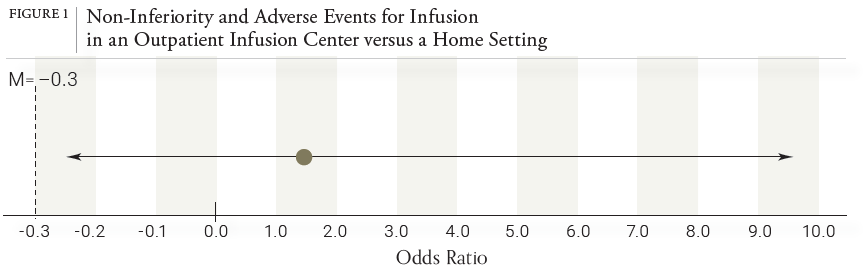
Multiple sclerosis (MS) is a progressive autoimmune inflammatory disorder causing demyelination and degeneration of the central nervous system, affecting 1 million people in the United States and about 2 million people worldwide.1 Natalizumab is a highly effective monoclonal antibody therapy for the treatment of relapsing-remitting multiple sclerosis (RRMS).1 It is usually delivered as 300 mg 1-hour intravenous infusion every 4 weeks.1 Natalizumab is generally well tolerated by patients, however, the safety profile of natalizumab in long term can be associated with a rare brain infection called progressive multifocal leukoencephalopathy (PML). Because of the potential for infusion-related reactions of natalizumab and the risk of PML, it poses a great inconvenience to patients requiring them to receive the infusions in an authorized infusion center as part of a restricted distribution program called the TOUCH® Prescribing Program.1 As part of the Coronavirus Disease 2019 (COVID-19) pandemic, patients and providers alike shared concerns about appointments in health care facilities that may increase immunocompromised patients’ exposure risk to COVID-19. As such, the manufacturer had requested, and received U.S. Food and Drug Administration (FDA) advice that enabled the manufacture to temporarily offer in-home infusion of natalizumab for patients with RRMS under the TOUCH® REMS program during the COVID-19 Public Health Emergency. Previous studies in countries outside of the U.S., and therefore regulated under different drug safety authorities, have described natalizumab home infusion models with positive results in safety, outcomes, and patient satisfaction.2,3
To support the provision of patient-centered care and provide home care as a safe option to patients with multiple sclerosis, it is necessary to develop a new model of care in the U.S. to deliver safe and effective therapy going forward.