In 2015, a male in his 30s diagnosed with chronic variable immunodeficiency (CVID), 1 of the more than 400 types of PI, presented to our services for home infusion of IVIG. During his treatment from 2015-2020, the monthly IVIG infusions were generally well tolerated, with no reports of severe adverse drug reactions (ADRs) or hospitalization related to home infusion therapy. The patient’s IVIG treatments included premedication with orally administered acetaminophen 650 mg and diphenhydramine 25 mg. After the first dose, hydration was added to the patient’s treatment plan to be administered post infusion. He received sodium chloride 0.9% 500 mL administered intravenously. Throughout treatment, the patient reported mild ADRs described as fatigue and lethargy during the infusion and for 36 hours post-infusion.
In 2020, the patient requested a transition from IVIG to SCIG to determine if SCIG could effectively treat his CVID without the ADRs he experienced using IVIG. As a result, the patient received SCIG 20% 10 gm weekly (40 grams per month, 504 mg/ kg/month). In addition, before each SCIG infusion, the patient received acetaminophen 650 mg and diphenhydramine 25 mg orally to prevent and treat SCIG ADRs.
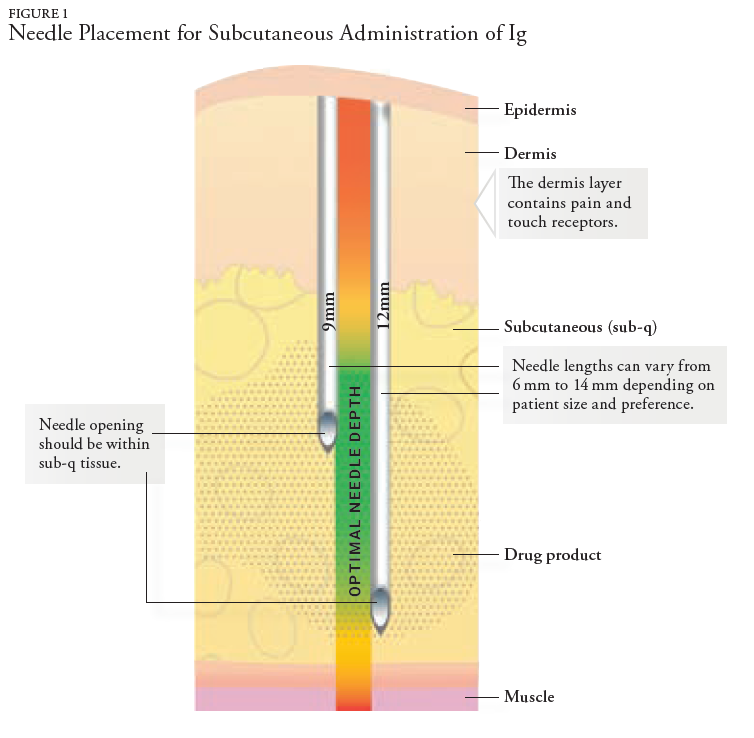
In late 2021, the patient noted numerous ADRs from his weekly SCIG infusions and stated he felt better on the monthly infusion of IVIG. The patient’s ADR list from SCIG included significant local swelling and pain, fatigue, lethargy, and complaints of back pain after each SC infusion. The ADRs were evaluated, and adjustments were made to slow the infusion. Additionally, the pharmacy changed the needle length from 9 mm to 12 mm to decrease local site reactions and ensure the distribution of Ig medication within the subcutaneous tissue. Despite making adjustments to eliminate or minimize the localized ADRs, the patient discussed a treatment plan with his prescriber to transition from weekly SCIG back to monthly IVIG infusions.
In December 2021, the patient was transitioned to IVIG 10% 30 gm every 4 weeks (357 mg/kg/4 weeks), administering the same brand of IVIG product previously used for IVIG treatment. He continued oral premedication with acetaminophen 650 mg and diphenhydramine 25 mg. Serum level monitoring of IgG reported: 494 mg/dL (March 2016), 823 mg/dL (April 2021), 1283 mg/ dL (July 2022), and 1347 mg/dL (January 2023). A comparison of the IV and SC doses showed that the patient’s monthly IVIG dose (30 gm) was 25% less than the total monthly SCIG dose (40 gm).
Since the transition from SCIG to IVIG, the patient denies ADRs. Nursing assessments during monthly infusion visits noted that the patient’s quality of life was improved after transitioning from SCIG to IVIG. The patient has remained stable on IVIG therapy since the transition. Patient preference was accommodated and resulted in improved patient satisfaction.