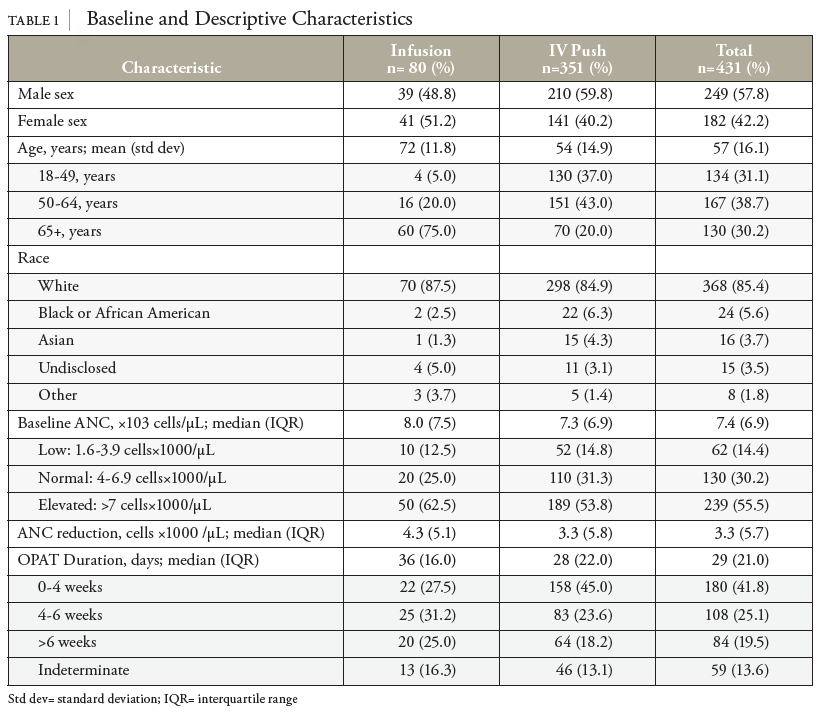
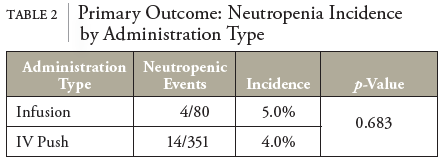
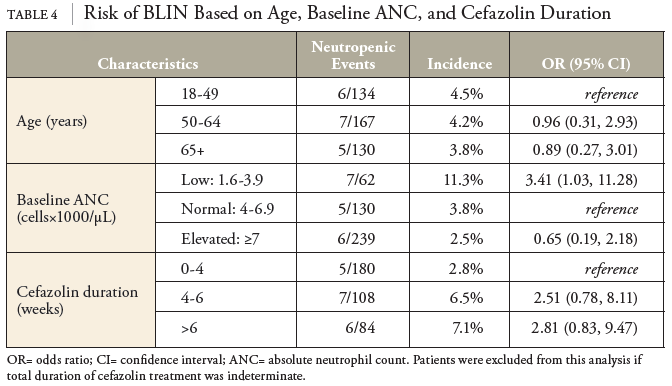
In this retrospective cohort study of patients receiving OPAT with cefazolin, baseline ANC was the greatest predictor for risk of neutropenic events. Patients with a baseline ANC between 1.6 and 3.9 were roughly 3.4 times more likely to experience a neutropenic event. In contrast to previous studies, no statistically significant differences in the incidence of neutropenia based on the method of delivery (IV push vs. intermittent infusion) were observed.
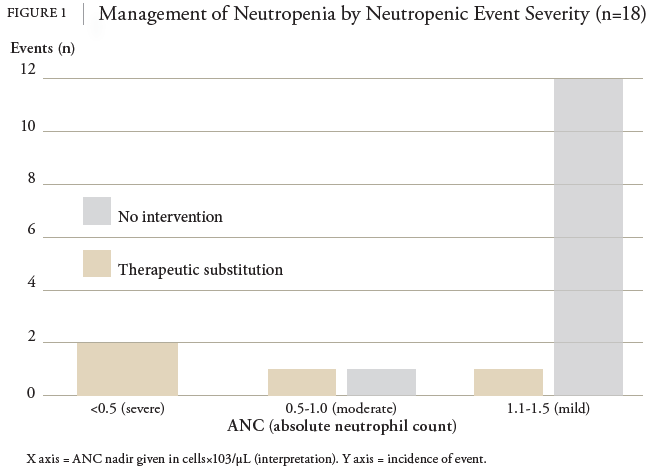
This study represents important progress for OPAT in home infusion. In the context of acute infection, neutrophil counts generally remain within normal ranges. Low neutrophil levels can indicate an underlying condition predisposing the patient to future neutropenic events. All events observed in this study were asymptomatic, and most were mild (ANC 1.5-1.1), requiring no intervention. Key cutoffs for neutropenia necessitating intervention are not well established and may be patient specific. Pharmacists play a critical role in monitoring labs, assessing risk for neutropenia, and relaying concerns to providers. Out of 18 incidents of BLIN, 5 led to an intervention. The pharmacist was responsible for monitoring for BLIN and reporting patient lab results to the prescriber. In conjunction with the prescriber, the pharmacist coordinated medication interventions. This finding supports the safe and effective practice of a pharmacist-led home infusion service for monitoring response to treatment in OPAT.
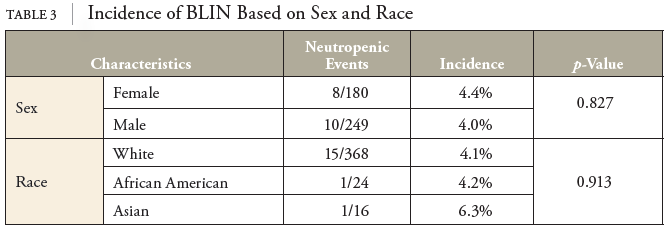
While a past medical history of antibiotic allergy was not included in the statistical analysis, investigators observed little correlation between allergy history and neutropenic events. If an immunologic mechanism is responsible for cefazolin-induced neutropenia, one might expect a prior antibiotic allergy to be a predisposing factor in the risk of developing antibiotic-induced neutropenia, particularly if the allergy were to a cephalosporin. This correlation was not observed, and since immune recognition has been associated with variations in R side chains of the beta-lactam ring, the research acknowledged that cefazolin does not share any similar or identified R1 or R2 side chains with other beta-lactams.
This study has several limitations. BLIN is rare and multifactorial; establishing a correlation with any 1 covariate is challenging. Thus, this study was underpowered to detect statistically significant differences in several key metrics. Statistically nonsignificant primary outcome results may be due to several factors. Patient preference for IVP administration makes adequately powering an IV infusion group challenging. Furthermore, age distributions between IVP and infusion groups must be considered. Generally, both geriatric and pediatric populations are more susceptible to adverse effects.19 This has been seen with BLIN, specifically in pediatric patients; although, as of now, no study has been identified showing older age to be associated with BLIN.13 Still, the potential for confounding with distributions of geriatric patients differing between groups (75% vs. 20%) must be considered.
Additionally, patients in the IVP group were educated to administer cefazolin over 10 minutes, on the conservative end of the IVP administration range. A recent study associated rapid IV push of cefepime with the rate of infusion administered IVP mediation over 3-5 minutes.12 The conservative approach for IVP administration in this study may indicate that a slower administration rate for IVP medications may mitigate the adverse effect. Furthermore, generalizability of these results to other sites may be limited by the observed patient characteristics. Overall, 85.4% of patients in this study were self-reported as white race. Thus, these results may translate differently to more racially diverse patient populations. Neutropenic events increase in frequency with increasing antibiotic durations. In most cases, neutropenic events occurred at or near the end of therapy. As a result, therapy was discontinued as planned, and ANC was rechecked at the follow-up appointment to confirm resolution, often 1-2 weeks later. However, ANC may have recovered well before the follow-up level was drawn. As a result, data on the duration of neutropenia was imprecise. Documentation of antibiotic stop dates for patients transferred to affiliated long-term care facilities was often not well documented within electronic health records. While these patients were not excluded from the study, they could not be included in logistic regression analysis without an appropriate duration of therapy.
Other covariates will be reassessed for correlations with BLIN in a follow-up study. Duration of treatment was of particular interest. Despite being underpowered to detect statistical differences, we observed odds ratios of 2.5 and 2.8 for durations of 4-6 weeks and 7+ weeks, respectively. If these results hold up to a larger sample size, this will confirm previous literature identifying longer treatment durations as a significant risk factor for BLIN. Notably, this study excluded patients with baseline ANCs below 1.5×103 cells/μL. Thus, additional studies are necessary to address optimal OPAT management for patients with baseline neutropenia or those receiving myelosuppressive chemotherapy.
Current recommendations for the management of BLIN are nonspecific and leave much to provider assessment based on ANC cutoffs and current risk of decompensation. Management strategies often start with careful laboratory monitoring in long-term betalactam treatment courses. In mild and asymptomatic BLIN, discontinuation of the offending agent is not always necessary. Watchful waiting and more frequent monitoring may prevent further decompensation. For patients at higher risk, transitioning to a beta-lactam containing an alternative R1 side chain is common. Finally, providers may utilize G-CSF to bolster the immune system and minimize infection risk; however, this was not observed in our study and is typically reserved for severe symptomatic neutropenia.11 Future development of a management algorithm for BLIN may hasten the continued success of OPAT in a home infusion setting.