The first case of COVID-19 in the U.S. was reported on January 21, 2020. Since then, there have been over 92 million reported cases and 1 million deaths.1 A combination of the high mortality rate and the debilitating effects of the virus has led to the development of various treatment options, including monoclonal antibodies (mAbs). The first mAb, muromonab-CD3 (OKT3), was produced in 1975 and fully licensed in 1986. Today, more than 100 mAbs have been approved by the U.S. Food and Drug Administration (FDA) and are used in treating various diseases and conditions, including cancer, chronic inflammatory diseases, transplant rejection, infectious diseases, and cardiovascular diseases.2 When used to treat COVID-19, mAbs assist in preventing viral progression in patients at risk for serious outcomes,3 especially those 65 and older with underlying comorbidities such as cardiovascular disease, obesity, diabetes, chronic kidney disease, and chronic lung disease.4 As reported by the National Institutes of Health (NIH), the major benefit from mAb therapy in the treatment of COVID-19 appears to be a reduction in the viral load, subsequently preventing hospitalizations and death.3 The NIH further states that the benefit of this type of therapy is that it is well-tolerated with minimal risks, with the most reported adverse events being injection site reactions and infusion-related reactions.3
On November 21, 2020, the FDA provided an emergency use authorization (EUA) for the mAbs REGEN-COV, which is casirivimab and imdevimab, administered together either intravenously or subcutaneously.5 This therapy was used for patients who tested positive for COVID-19 and were at high risk for progressing to severe COVID-19, hospitalization, or both, and had mild-to-moderate COVID-19 symptoms.5 A phase 3 adaptive trial was conducted on REGEN-COV and showed that COVID-19 related hospitalizations or death from any cause occurred in 18 of 1,355 (1.3%) patients in the REGEN-COV group compared to 62 of 1,341 (4.6%) patients in the placebo group.6 The trial concluded that REGEN-COV reduced the risk of COVID-19-related hospitalizations or death from any cause, resolved symptoms, reduced the SARS-CoV-2 viral load more rapidly than placebo.6
To ease access to treatment and due to the contagious nature of COVID-19, many patients and care takers gravitated to home health care. Furthermore, with changes in the Centers for Medicare and Medicaid Services (CMS) reimbursement for home COVID-19 treatment, home infusion providers could administer REGEN-COV in the home. As a novel COVID-19 treatment, the National Home Infusion Foundation (NHIF) retrospectively collected and analyzed data from the REGEN-COV patient charts. From the results of this analysis, it was reported that home infused REGEN-COV achieved comparable outcomes to other sites of care and provided improved access to treatment for SARS-COV-2 infection.7 Furthermore, it was concluded that home infusion providers played an essential role in reducing exposure, saving lives, and reducing hospitalizations.7
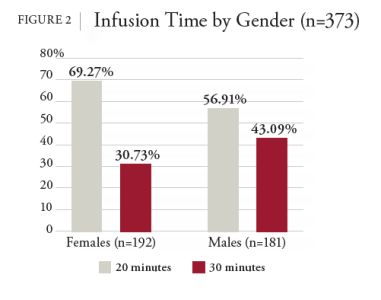
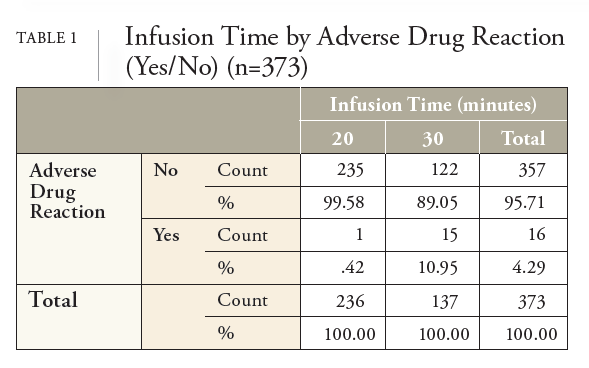
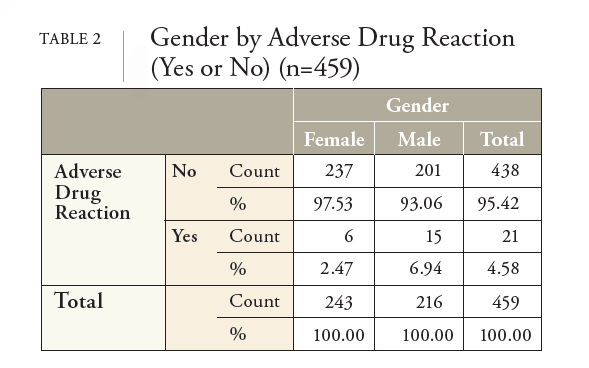
NHIF decided to conduct additional analysis of the REGEN-COV data after noting the adverse drug reaction (ADR) recommendation in the medical literature that advocated for reporting systems to expand their focus to include genderrelated factors to understand, prevent, or reduce the occurrence of ADRs in all people.8 Without these reporting systems, ADRs that are more common among certain groups, such as women, may remain undetected for years, increasing the possibility of unanticipated risks.8 It was further stated that research is needed to identify the relationships between gender-related factors in the occurrence and reporting of ADRs to adequately detect and prevent ADRs.8 Subsequently, this study aimed to answer the research question, “Does a significant difference exist between men and women and the rate of ADRs from REGENCOV?” If a significant difference does exist, what confounding variables might have caused the difference between the genders, such as patient age, infusion time, vaccination status, and the number of days between the onset of COVID-19 symptoms and the first dose of REGEN-COV.
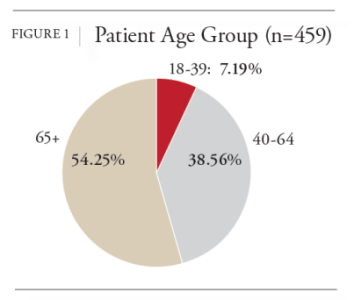
Prior to conducting this investigation, literature on gender differences and ADRs was searched. A recent study that reviewed 33,147 patient charts with an ADR-related hospital admission between 2005 and 2017 determined that women accounted for 55.72% of ADR-related hospital admissions while men accounted for 44.28%.9 It was also revealed a significant difference between the mean age of the men and women (Women = 72.1 years, Men = 71.3 years) and in the types of therapies that men and women were using. Both variables are known to skew the rate of ADRs in patients, evident by research that shows that the elderly are thought to be predisposed to ADRs.10 Another ADR study that included 513,608 patients who were prescribed a newly marketed drug, concluded that women tend to have a 1.5-1.7 times higher risk of developing ADRs.11 These results are in line with conclusions from other researchers.12-14 The only study that addressed the rate of ADRs in home infused patients was a 2022 study by NHIF that had a sample size of 6,045 and concluded that the rate of ADRs that result in discontinuation from therapy is 0.33%.15 Unfortunately, this study did not report the ADR rate by gender. Overall, the research on gender and ADRs concludes that women have a higher rate of ADRs than men, though most studies did not control for age and therapy differences between the genders.
Research Question
Does a significant difference (p ≤ .05) exist between men and women and the rate of ADRs from intravenous infusion of REGEN-COV in the home setting? If a significant difference does exist, what other factors might have caused the difference between the genders, such as patient age, intravenous infusion rate, vaccination status, and the number of days between the onset of COVID-19 symptoms and first dose of REGEN-COV.