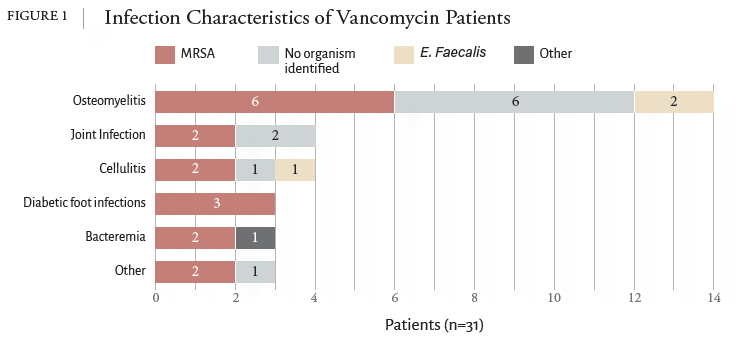
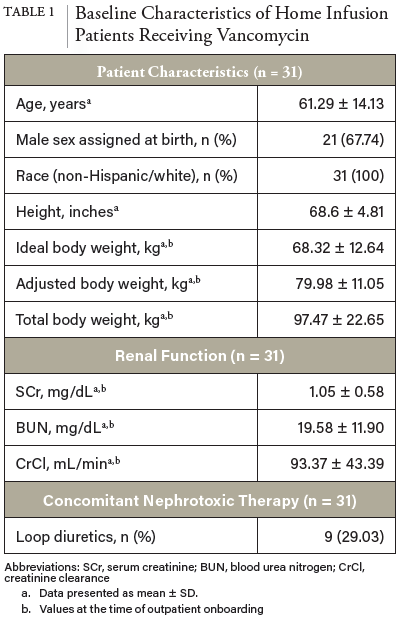
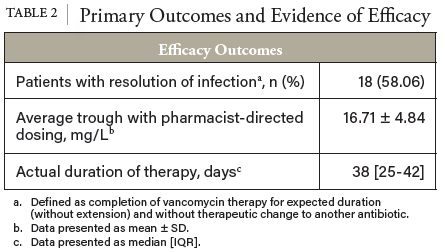
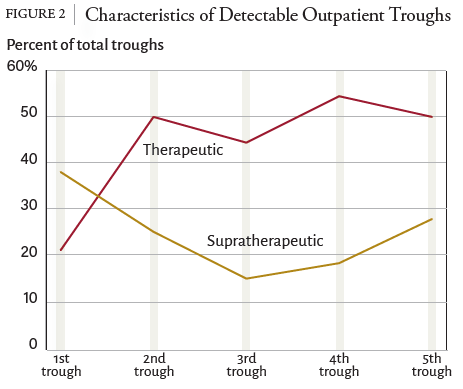
1. Filippone EJ, Kraft WK, Farber JL. The Nephrotoxicity of Vancomycin. Clin Pharmacol Ther. 2017;102(3):459-469. doi:10.1002/cpt.726
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Marquis KA, DeGrado JR, Labonville S, Kubiak DW, Szumita PM. Evaluation of a Pharmacist-Directed Vancomycin Dosing and Monitoring Pilot Program at a Tertiary Academic Medical Center. Ann Pharmacother. 2015;49(9):1009-1014. doi:10.1177/1060028015587900
4. Phillips CJ, Gordon DL. Pharmacist-led implementation of a vancomycin guideline across medical and surgical units: impact on clinical behavior and therapeutic drug monitoring outcomes. Integr Pharm Res Pract. 2015;4:145-152. doi:10.2147/IPRP.S92850
5. Hovey SW, Jacobson JL, Welsh KM, Vu BN. Implementation of a Pharmacist-Driven Vancomycin and Aminoglycoside Dosing Service in a Pediatric Hospital. The Journal of Pediatric Pharmacology and Therapeutics. 2022;27 (4): 340–346. doi: 10.5863/1551-6776-27.4.340
6. Momattin H, Zogheib M, Homoud A, Al-Tawfiq JA. Safety and Outcome of Pharmacy-Led Vancomycin Dosing and Monitoring. Chemotherapy. 2016;61(1):3-7. doi:10.1159/000440607
7. Fodero KE, Horey AL, Krajewski MP, et al. Impact of an Antimicrobial Stewardship Program on Patient Safety in Veterans Prescribed Vancomycin. Clin Ther, 2016;38(3):494-502. doi: 10.1016/j.clinthera.2016.01.001
8. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-c184. doi:10.1159/000339789
9. Perazella MA, Rosner MH. Drug-Induced Acute Kidney Injury. Clin J Am Soc Nephrol. 2022;17(8):1220-1233. doi:10.2215/CJN.11290821
10. Neely MN, Youn G, Jones Bet al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014; 58(1):309-316. doi: 10.1128/AAC.01653-13
11. Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2016;11(12):2132-2140. doi:10.2215/CJN.05920616