1. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Veklury® (Remdesivir), US Food and Drug Administration revised 10/2020
2. Veklury® (Remdesivir) Prescribing Information, Gilead Sciences, Inc. 2020
3. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 Final Report. N Engl J Med. 2020;383(19):1813- 1826.
4. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(11):1048- 1057.
5. Goldman JD, Lye DB, Hui DS, et al. Remdesivir for 5 or 10 days in Patients with Severe COVID. N Engl J Med 2020; 383:1827-1837DOI: 10.1056/NEJMoa2015301
6. WHO Solidarity Trial Consortium; Pan H, Peto R, Henao-Restrepo AM et al. Repurposed Antiviral Drugs for COVID-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. PMID: 33264556; PMCID: PMC7727327.
7. COVID-NMA: Living evidence synthesis. Accessed from: https://covidnma.com/living_data/index.php?comparison=14. Updated July 30, 2021. References
8. WHO Solidarity Trial Consortium; Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated metaanalyses. Lancet 2022 May 21;399(10339):1941-1953.
9. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Accessed from: https://www.idsociety.org/practiceguideline/covid-19-guideline-treatment-andmanagement/. Updated May 16, 2021.
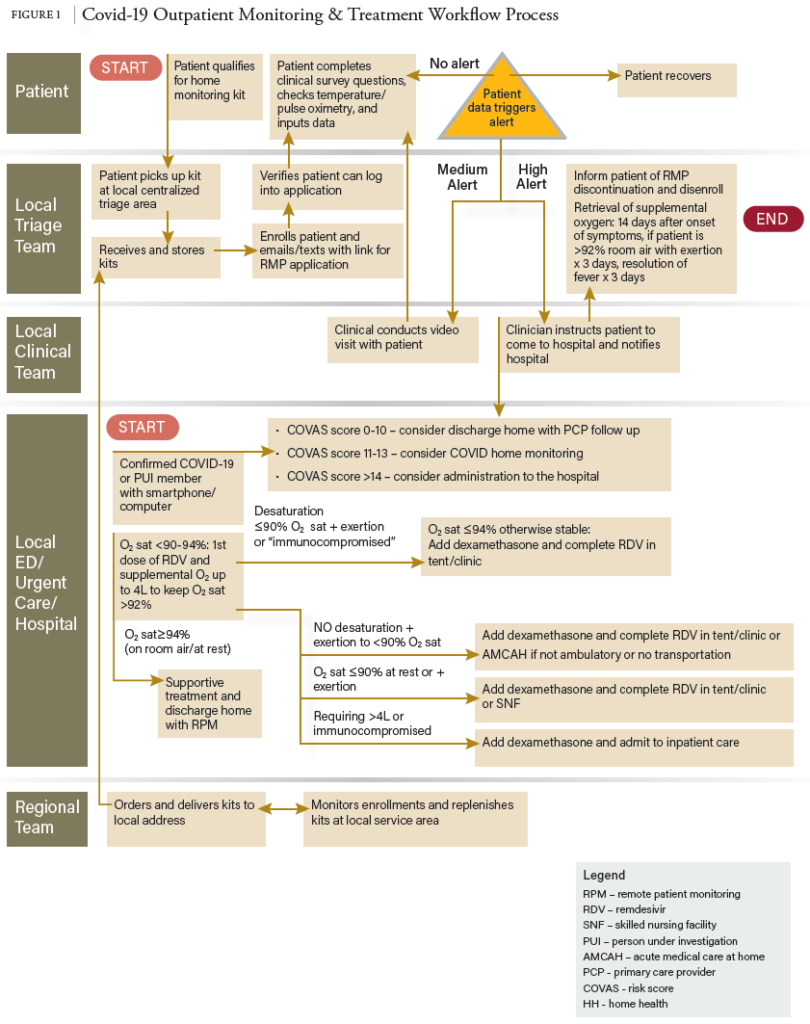
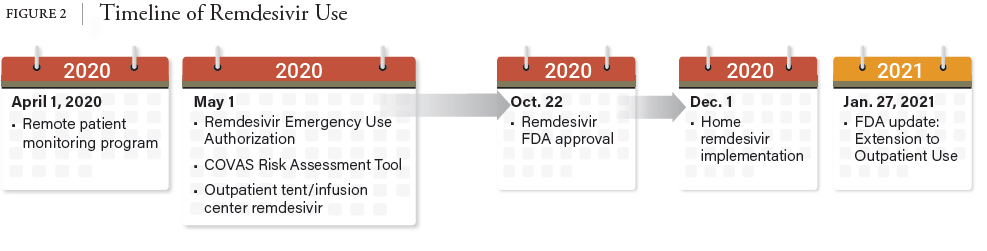
10. Federal Response to COVID-19: Therapeutics Clinical Implementation Guide. Outpatient Administration Guide for Health care Providers (hhs.gov)
11. Fox E, Shah M, Vinik R et al. Developing state-wide remdesivir use criteria. Am J Health-Syst Pharm. 2021; 78:732-735. DOI 10.1093/ajhp/zxba009
12. Milla-Godoy GC, Prasongdee K, Cristancho C, Poloju A, Barbosa F, Treadwell T. A Tale of Two Surges: Differences in Outcomes in the COVID-19 Pandemic in a Community Teaching Hospital in Massachusetts. Cureus. 2022 Jan 24;14(1):e21547. doi: 10.7759/ cureus.21547.
13. Centers for Medicare & Medicaid Services. Acute Hospital Care at Home Program. https://www.dhcs.ca.gov/Pages/Acute-Hospital-Care-at-Home-Program.aspx. Accessed 03/9/23
14. Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012 Summer;16(3):37-41. doi: 10.7812/ TPP/12-031. PMID: 23012597; PMCID: PMC3442759.