Menu
Volume 1, Issue 2
>
A Retrospective Cohort Study of Vancomycin Dose Reductions Among Home Infusion Patients Post-Hospitalization
Background: Patients treated with intravenous (IV) vancomycin in the hospital often require outpatient parenteral antimicrobial therapy (OPAT) after discharge for the continuation of therapy. Despite vigilant monitoring, nephrotoxicity is a common adverse drug event associated with vancomycin in the home infusion setting.
Methods: This multi-center retrospective cohort study included adult patients from the North Central United States receiving trough-based IV vancomycin dosing for osteomyelitis between April 1, 2021, and June 30, 2021. The primary objective was to determine the percentage of patients requiring vancomycin dose reductions upon transition from an inpatient setting to home infusion services. Secondary outcomes evaluated the incidence of acute kidney injury (AKI) and rehospitalization rates due to AKI.
Results: A total of 94 patients were included and evaluated for dose reductions of vancomycin. Of these, 47 (50%) patients required dose reductions throughout therapy, with 24 (51%) reductions occurring within the first 7 days post-hospitalization. Nine (9.5%) patients developed AKI from vancomycin within 2-7 days post-hospitalization, and 4 (4.3%) patients required readmission due to AKI.
Conclusions: Most patients in this study required vancomycin dose reductions within the first 7 days post-hospitalization, indicating the importance of careful monitoring upon transition to home infusion services. Patients receiving vancomycin dose reductions before hospital discharge did not experience AKI or rehospitalization. Empiric vancomycin dose modifications may be reasonable with proper clinical judgment but should be monitored closely to ensure therapeutic drug levels and patient safety.
Keywords: Home infusion, vancomycin, outpatient parenteral antimicrobial therapy, therapeutic drug monitoring, nephrotoxicity, MRSA
Vancomycin is a glycopeptide antibiotic with bactericidal activity commonly used to treat gram-positive infections, including methicillin-resistant Staphylococcus aureus (MRSA).1 Intravenous (IV) vancomycin requires extensive clinical monitoring in both community and health care settings to maintain efficacy and limit toxicity. Parenteral antibiotics are often used to treat severe infections and can be administered in the home setting with proper patient education.2 However, a significant concern of vancomycin use is the incidence of nephrotoxicity.
Until recently, therapeutic drug monitoring (TDM) for vancomycin has been centered on maintaining trough concentrations between 15 and 20 mg/L for severe MRSA infections.3 Although trough monitoring has been heavily integrated into clinical practice over the years, current data correlates the risk of acute kidney injury and supratherapeutic vancomycin trough levels.3-4 Published literature regarding the incidence of vancomycin-induced AKI is more established in acute care settings. In a meta-analysis by van Hal and colleagues, vancomycin-associated AKI varied from 5 to 43%. Most episodes of AKI developed between 4 and 17 days after initiation of vancomycin therapy.4
Upon hospital discharge, patients often require home infusion services to continue therapy. Hydration status between the acute care and home settings may impact drug metabolism and clearance, posing a risk to patient safety after hospital discharge. Vancomycin clearance is dependent on the glomerular filtration of the kidneys; therefore, renal dysfunction slows the excretion of vancomycin and is usually a reversible process.1 Home infusion pharmacists perform clinical monitoring and provide therapeutic recommendations based on renal function and vancomycin serum concentrations to ensure patient safety.
Currently, no published literature addresses the incidence of vancomycin-induced nephrotoxicity in this setting. The primary objective of this study was to determine the percentage of patients requiring vancomycin dose reductions upon transition from the inpatient setting to home infusion services, as well as throughout therapy in the home setting. Dose reductions were noted on days 0, 1-7, 8-14, and >14 based on clinical judgment and laboratory values, such as serum creatinine and vancomycin trough levels. Secondary outcomes evaluated the incidence of AKI and rates of rehospitalization due to AKI. Results of this study may indicate whether an empiric dose reduction before starting home infusion services would prevent the incidence of vancomycin-induced nephrotoxicity following hospitalization.
This multi-center retrospective cohort study included patients from the North Central United States. Patients 18 years and older who received trough-based IV vancomycin dosing for osteomyelitis between April 1, 2021, and June 30, 2021, were evaluated for inclusion. This population was selected to target vancomycin trough levels between 15 to 20 mg/L, as these levels correlate with vancomycin-induced AKI.3-4 Patients were excluded if vancomycin was initiated in the outpatient setting, received vancomycin dosing based on Area Under the Curve/Minimum Inhibitory Concentration (AUC/MIC), or concomitant use of piperacillin-tazobactam.
Patient electronic health records were retrospectively reviewed for hospital discharge orders, laboratory results, home infusion-related assessments, and interventions. For the primary outcome analysis, vancomycin dosing regimens and corresponding trough values were analyzed throughout therapy to determine the need for dose reductions or extended intervals between doses. Electronic health record review also determined the number of patients developing AKI and those requiring hospital readmission due to AKI. Based on Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, AKI was defined as an increase in serum creatinine of ≥0.3 mg/dL within 48 hours or 1.5 times increase from baseline within the last seven days.5 For this study, baseline renal function was based on hospital discharge laboratory values.
The research involved secondary data analysis where the data set was deidentified before analysis and recorded in a manner where the resulting data contained no information that could be linked directly or indirectly to the identity of the patients. This study was determined as exempt from IRB review.
A total of 141 patients were screened for study enrollment. Of these, 94 patients met inclusion criteria. Forty-four patients were excluded because vancomycin was initiated in the outpatient setting rather than continuing therapy post-hospitalization. Two patients were excluded due to concomitant use of piperacillin-tazobactam and 1 patient who received AUC-based dosing. Included patients were majority male (73.4%) and had an average age of 63.37 years (SD=15.51). Patient ages ranged from 22-97 years old.
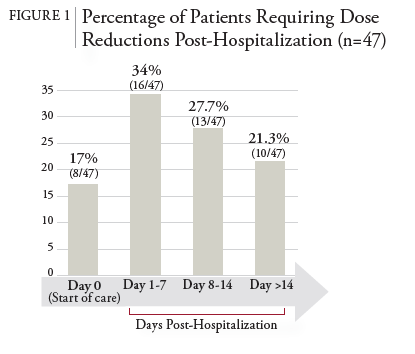
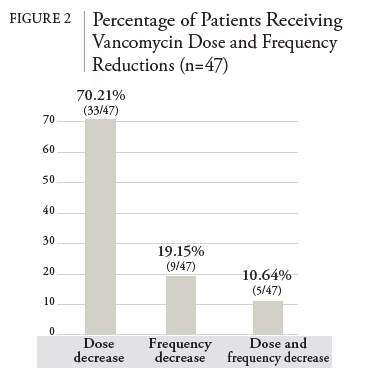
Patients who met inclusion criteria were observed for the primary and secondary endpoints. 47 (50%) patients required dose reductions throughout therapy. Most vancomycin dose reductions occurred within 7 days post-hospitalization, with 24 (51%) total reductions occurring during this period. The age range of the 47 patients with dose reductions was 40 to 84 years old. Eight (17%) patients had empiric dose reductions on day 0 before starting home infusion services. Of note, 3 regimens were empirically modified to longer dosing intervals (e.g., from every 18 to every 24 hours) by home infusion pharmacists based on clinical judgment for ease of administration and increased adherence in the home setting. Inpatient pharmacists performed the other 5 interventions for dose reductions on day 0 before hospital discharge. An additional 13 (27.7%) regimens were dose reduced on days 8-14 and 10 (21.3%) regimens on days >14. The primary outcome results are summarized in Table 1 and Figure 1. Figure 2 demonstrates how vancomycin reductions occurred by dose, frequency, or both.
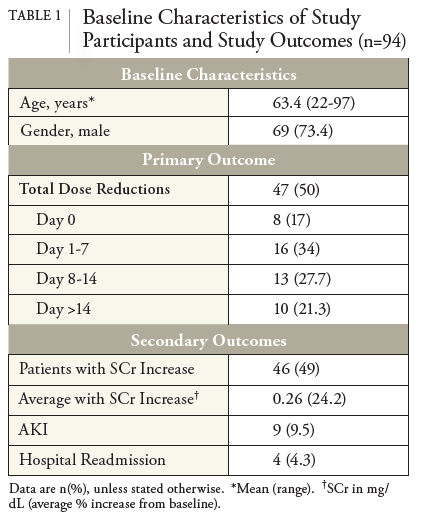
Overall, 46 (49%) patients experienced an increase in serum creatinine on therapy, with an average increase of 0.26 mg/dL (SD=0.27) from baseline. A total of 9 (9.5%) patients developed AKI from vancomycin within 2-7 days post-hospitalization. These patients were between 40 and 85 years old. Three of the 9 patients developed AKI within 48 hours upon transitioning to home infusion services. Four (4.3%) patients required hospital readmission due to AKI. None of the patients with vancomycin dose reductions on day 0, before home infusion services, experienced AKI or rehospitalization due to AKI. Vancomycin dose increases occurred in 2 patients with subtherapeutic and therapeutic trough levels despite worsening renal function. In one case, the patient developed a notable AKI within 48 hours of transitioning to home infusion services, followed by a dose increase. Secondary outcome results can be seen in Table 1.
Upon transition to the home infusion setting, empiric dose reductions of vancomycin are based on clinical judgment and feasibility of home administration. Before hospital discharge, inpatient pharmacists are involved with vancomycin dosing essentially based on renal function and TDM. After discharge, patients are further evaluated by home infusion pharmacists for appropriateness of the vancomycin indication and dosing regimen.
For severe MRSA infections, current guidelines recommend AUC/MIC monitoring to improve patient safety and reduce rates of nephrotoxicity. One approach to accomplish AUC-based therapy involves using Bayesian dose-optimizing software, which requires minimal pharmacokinetic (PK) sampling.3 Alternatively, multiple serum concentrations are collected to calculate AUC using analytic PK equations.6 Despite increased utilization of AUC/MIC-based vancomycin dosing for severe MRSA infections, this monitoring strategy has not been widely adapted in the home infusion setting. Due to the cost limitations of acquiring Bayesian software, trough monitoring is still commonly used in the home infusion setting.
Throughout vancomycin therapy, 50% of patients in this study required dose reductions, most occurring within 7 days post-hospitalization. Patients are at an increased risk of dehydration, leading to AKI immediately post-hospitalization.7 The cessation of IV hydration and increased ambulation causing fluid mobilization may contribute to hydration status following hospitalization. Compared to the inpatient setting, these factors contributing to dehydration in the home may alter renal function, thus changing the predicted vancomycin PK. Upon transition to home infusion services, patients receiving vancomycin dose reductions on day 0 did not experience AKI or rehospitalization during therapy. This finding suggests empirically reducing vancomycin doses post-hospitalization for continuation with home infusion services may improve patient safety regarding nephrotoxicity while sustaining efficacy. A concern with empiric vancomycin dose reductions is the potential for suboptimal trough levels leading to antimicrobial resistance. With known MRSA infection, it is essential to maintain levels within the therapeutic range.
Of the patients who experienced nephrotoxicity, the most common time for dose reductions was between days 8 and 14. In this population, the delay in dose reductions was often due to therapeutic vancomycin trough levels in the setting of serum creatinine values trending upward. In one case, the vancomycin dose was increased due to subtherapeutic trough values in worsening renal function. This led to drug accumulation and nephrotoxicity, reinforcing the importance of various factors influencing vancomycin pharmacokinetics.
Limited literature is available on vancomycin-induced nephrotoxicity in the home infusion setting. Limitations of this study include the retrospective study design and the small sample size. In addition, a comprehensive past medical history is not always available when providing outpatient parenteral antimicrobial therapy (OPAT) after hospital discharge. It was unknown whether patients were predisposed to nephrotoxicity due to a history of chronic kidney disease (CKD) or CKD related to diabetes. More extensive studies expanding to different regions of the United States, as well as the inclusion of other severe MRSA infections requiring prolonged treatment courses, such as bacteremia, endocarditis, and meningitis, may be beneficial.
Half of the study population required dose reductions within the first week of home infusion services. Patients may experience a shift in fluid status post-hospitalization, causing dehydration and altered renal function. Empirically reducing vancomycin regimens may correlate with a decreased incidence of AKI as patients transition from the hospital to home infusion services to continue therapy. Patients who received dose reductions on day 0, before starting home infusion services did not experience nephrotoxicity or hospital readmission due to AKI.
Practitioners should continue closely monitoring all vancomycin dose modifications to ensure optimal therapeutic drug levels and maximize patient safety. As clinical evidence continues to evolve, the implementation of AUC/MIC-based vancomycin dosing rather than trough-based dosing alone will enhance patient safety regarding the incidence of AKI.3,6 Further research with larger sample sizes is needed to confirm the results of this study.
1. Vancomycin [Package Insert]. Baudette, MN: ANI Pharmaceuticals, Inc. Jan. 2017.
2. Matthews PC, Conlon CP, Berendt AR et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007; 60(2):356–362.
3. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review of the American Society of Health-System Pharmacists, the Infectious Diseases Society by America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm. 2020; 77: 835-864.
4. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013; 57(2):734- 744.
5. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012; 2: 1-138.
6. Nix DE, Davis LE, Matthias KR. The relationship of vancomycin 24-hour AUC and trough concentration. Am J Health-Syst Pharm. 2022; 79:534-539.
7. Wakefield BJ, Mentes J, Holman JE, et al. Postadmission Dehydration: Risk Factors, Indicators, and Outcomes. Rehabilitation Nursing. 2009; 34(5):209–216.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |