1. Polinski JM, Kowal MK, Gagnon M, Brennan TA, Shrank WH. Home infusion: Safe, clinically effective, patient preferred, and cost saving. Healthc (Amst). 2017;5(1-2):68-80. Epub 20160429. doi: 10.1016/j.hjdsi.2016.04.004. PubMed PMID: 28668202.
2. Haines DJ, Simpson M. Rate of discontinuation from home infusion therapy due to adverse drug reactions and unplanned hospitalization – A pilot study. INFUSION NHIF Research Supplement, September/ October 2021; 2-7.
3. Sullivan C, Haines DJ. Setting the bar – Establishing industry standards through benchmarking. INFUSION, July/August 2018; 23(4):26-33.
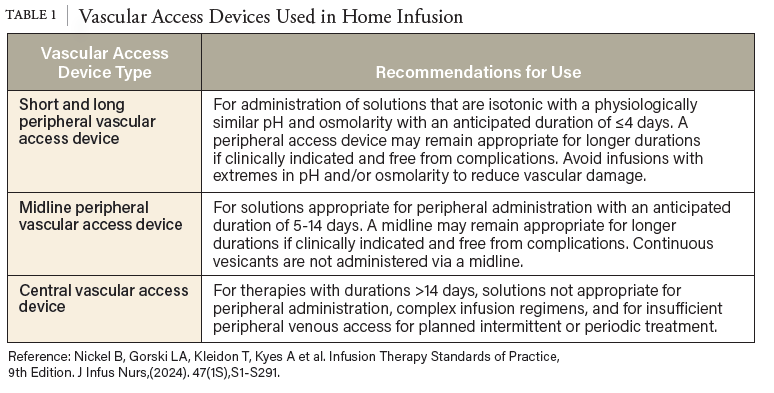
4. Nickel B, Gorski L, Kleidon T, Kyes A, DeVries M, Keogh S, et al. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024;47(1S Suppl 1):S1-S285. doi: 10.1097/ NAN.0000000000000532. PubMed PMID: 38211609.
5. Manrique-Rodriguez S, Heras-Hidalgo I, Pernia-Lopez MS, Herranz-Alonso A, Del Rio Pisabarro MC, Suarez-Mier MB, et al. Standardization and Chemical Characterization of Intravenous Therapy in Adult Patients: A Step Further in Medication Safety. Drugs R D. 2021;21(1):39-64. Epub 20201221. doi: 10.1007/s40268-020- 00329-w. PubMed PMID: 33346878; PubMed Central PMCID: PMC7937591.
6. Kovacevich DS, Corrigan M, Ross VM, McKeever L, Hall AM, Braunschweig C. American Society for Parenteral and Enteral Nutrition Guidelines for the Selection and Care of Central Venous Access Devices for Adult Home Parenteral Nutrition Administration. JPEN J Parenter Enteral Nutr. 2019;43(1):15-31. Epub 20181019. doi: 10.1002/jpen.1455. PubMed PMID: 30339287.
7. Norris AH, Shrestha NK, Allison GM, Keller SC, Bhavan KP, Zurlo JJ, et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi: 10.1093/cid/ciy745. PubMed PMID: 30423035.
8. van den Bosch CH, Spijkerman J, Wijnen M, Hovinga I, Meyer- Wentrup FAG, van der Steeg AFW, et al. Central venous catheterassociated complications in pediatric patients diagnosed with Hodgkin lymphoma: implications for catheter choice. Support Care Cancer. 2022;30(10):8069-79. Epub 20220701. doi: 10.1007/s00520- 022-07256-3. PubMed PMID: 35776186; PubMed Central PMCID: PMC9512752.
9. Yeow M, Soh S, Yap R, Tay D, Low YF, Goh SSN, et al. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1184-91 e8. Epub 20220330. doi: 10.1016/j.jvsv.2022.03.007. PubMed PMID: 35367407.
10. Capozzi VA, Monfardini L, Sozzi G, Armano G, Butera D, Scarpelli E, et al. Peripherally Inserted Central Venous Catheters (PICC) versus totally implantable venous access device (PORT) for chemotherapy administration: a meta-analysis on gynecological cancer patients. Acta Biomed. 2021;92(5):e2021257. Epub 20211103. doi: 10.23750/abm. v92i5.11844. PubMed PMID: 34738565; PubMed Central PMCID: PMC8689318.
11. Christensen LD, Holst M, Bech LF, Drustrup L, Nygaard L, Skallerup A, et al. Comparison of complications associated with peripherally inserted central catheters and Hickman catheters in patients with intestinal failure receiving home parenteral nutrition. Six-year follow up study. Clin Nutr. 2016;35(4):912-7. Epub 20150728. doi: 10.1016/j.clnu.2015.06.009. PubMed PMID: 26269383.